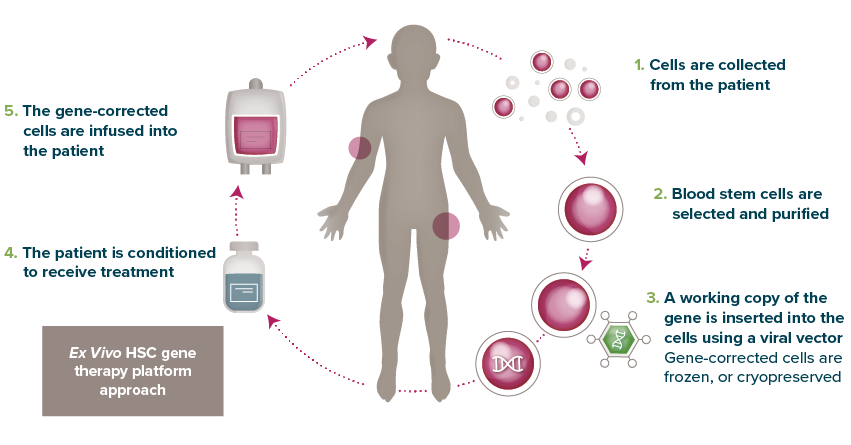
Gene therapy comes in many forms: Here’s my insight and overview of Orchard’s HSC gene therapy approach
By Bobby Gaspar, M.D., Ph.D.
September 8th, 2020

The site uses cookies to provide you with a more responsive and personalized service. By using this site, you agree to our use of cookies as set out in our cookie policy.
Please read our privacy policy and cookie policy for more information on the cookies we use and how to delete or block the use of cookies.
Please read our privacy policy and cookie policy for more information on the cookies we use and how to delete or block the use of cookies.
Notice:
Orchard Therapeutics is not responsible for the content of external Internet sites.
If you wish to continue, click “Continue”, or otherwise click “Stay on page”